Wednesday, December 17, 2025
What Is Dissolved Oxygen (DO) and Why It Matters for Water Health
What Is Dissolved Oxygen?
Dissolved oxygen is oxygen that exists in water in molecular form, not as bubbles you can see which are pockets of many molecules stuck together. It’s measured in milligrams per liter (mg/L) or parts per million (ppm). Aquatic life and plant roots rely on DO for respiration — the process of converting nutrients into energy.
Maintaining proper DO levels is critical for:
Aquatic species like fish and shrimp
Plant roots in hydroponics and soil systems
Aerobic microbial communities that break down waste
Aquatic life and plants cannot directly use "bubbles", only actually dissolved oxygen molecules. That's why simply introduction bubbles into water is only half of the deal. Another part is to dissolve bubbles into the water.
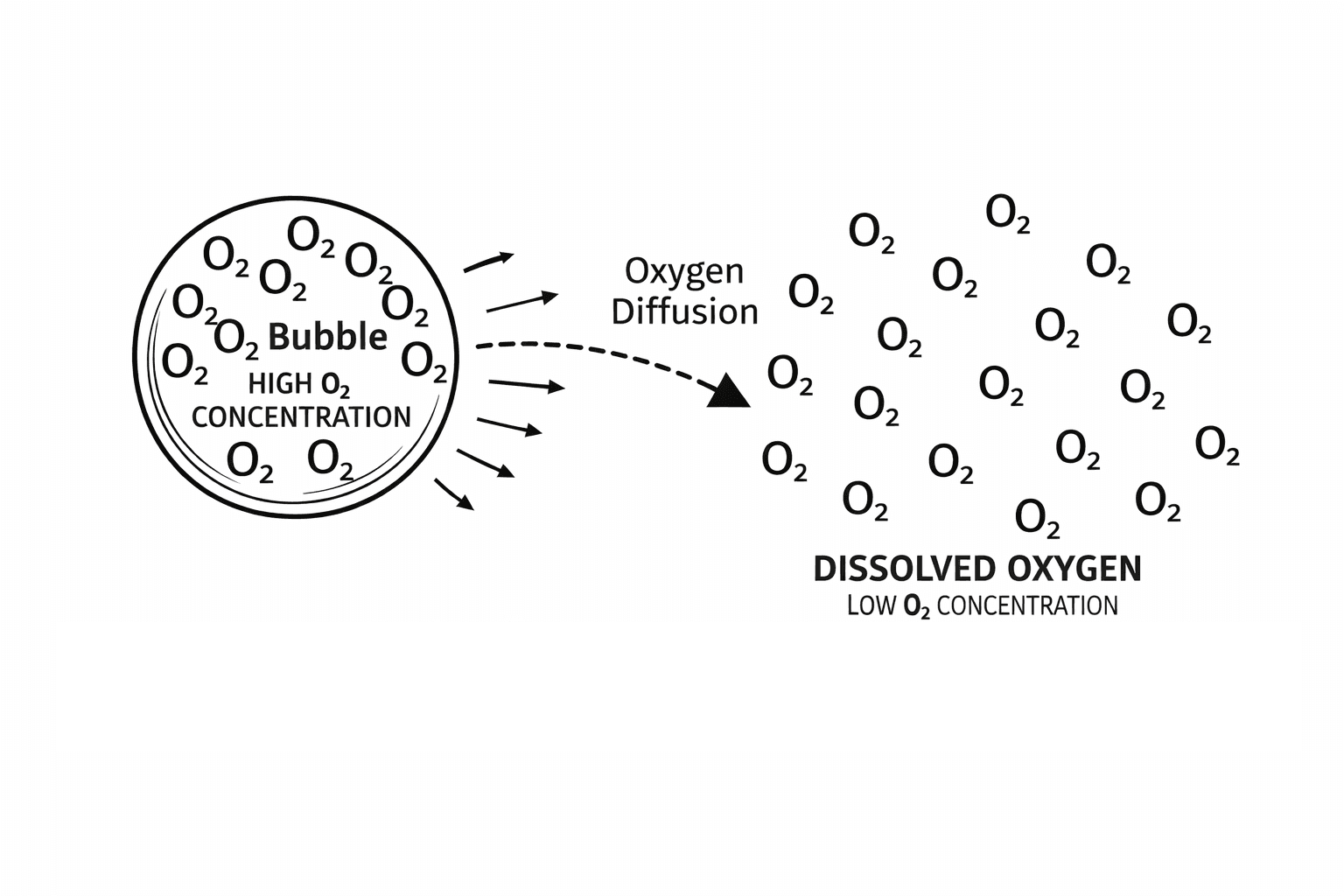
How Oxygen from Bubbles Actually Dissolves into Water
When a bubble is in the water, oxygen molecules at the bubble’s surface move into the surrounding water in a process called diffusion. This happens because water can hold a certain amount of dissolved oxygen, and molecules naturally move from areas of high concentration (inside the bubble) to low concentration (the water). Smaller bubbles have a much larger surface-area-to-volume ratio, which allows more oxygen molecules to transfer into the water before the bubble reaches the surface and escapes. Nanobubbles are particularly effective because their tiny size keeps them suspended in water for days, slowly releasing oxygen and maintaining high dissolved oxygen levels over time, whereas regular bubbles rise in matter of seconds giving no time for the diffusion to happen.
What Affects Dissolved Oxygen Levels?
Temperature and DO Solubility
Water temperature has a direct impact on how much oxygen it can hold. Cooler water holds more oxygen; warmer water holds less. That means DO naturally declines as water warms — creating stress in irrigation reservoirs, fish tanks, and recirculating systems.
Want to learn more? Read “Why Warm Water Holds Less Dissolved Oxygen” for a full explanation of this thermodynamic effect and practical implications in agricultural systems.
Maximum DO Depends on Gas Source
Not all oxygenation is the same. If you aerate with regular air, DO will top out around a certain level because air only contains about 21% oxygen. Nanobubbles filled with pure oxygen can drive DO far beyond what air injection can achieve, especially when dissolved oxygen needs are high.
Why DO Is Critical for Plants and Roots
Plant roots need oxygen just like leaves need sunlight. Without it:
Respiration slows
Nutrient uptake drops
Root diseases become more likely
Dissolved oxygen levels near the root zone influence crop yield, stress tolerance, and overall performance.
This connection is explored in “How Nanobubbles Enhance Root Oxygenation and Nutrient Absorption in Plants.”
DO and Aquaculture
Fish and other aquatic livestock are especially sensitive to DO. Rising temperatures reduce DO just when fish metabolism increases — a double stress that can slow growth and raise disease risk.
Learn more in “Rising Temperatures, Oxygen Stress, and the Role of Nanobubbles in Fish Farming.”
Biological Balance: DO vs. Pathogens
Low oxygen doesn’t just stress desirable organisms — it also encourages harmful pathogens like Pythium in hydroponic and irrigation systems, which thrive in oxygen-poor conditions. Managing DO is one of the most effective preventative measures.
How Nanobubbles Change the DO Game
Conventional aeration methods (large bubbles from stones or diffusers) often lose most oxygen back to the atmosphere. Nanobubble technology — especially when paired with high-purity oxygen — introduces ultrafine bubbles that:
Stay suspended for longer
Increase gas–liquid contact surface area
Release oxygen gradually into the water
This leads to higher, more stable dissolved oxygen with lower energy use.
Wrapping Up: DO as a Water Health Control
Dissolved oxygen isn’t just a measurement — it’s a control lever for healthier water systems:
Better plant root performance
Stronger aquaculture growth
Higher aerobic microbial activity
Lower pathogen risk
Whether you’re managing irrigation, aquaculture, or recirculating systems, understanding and optimizing DO is foundational.



